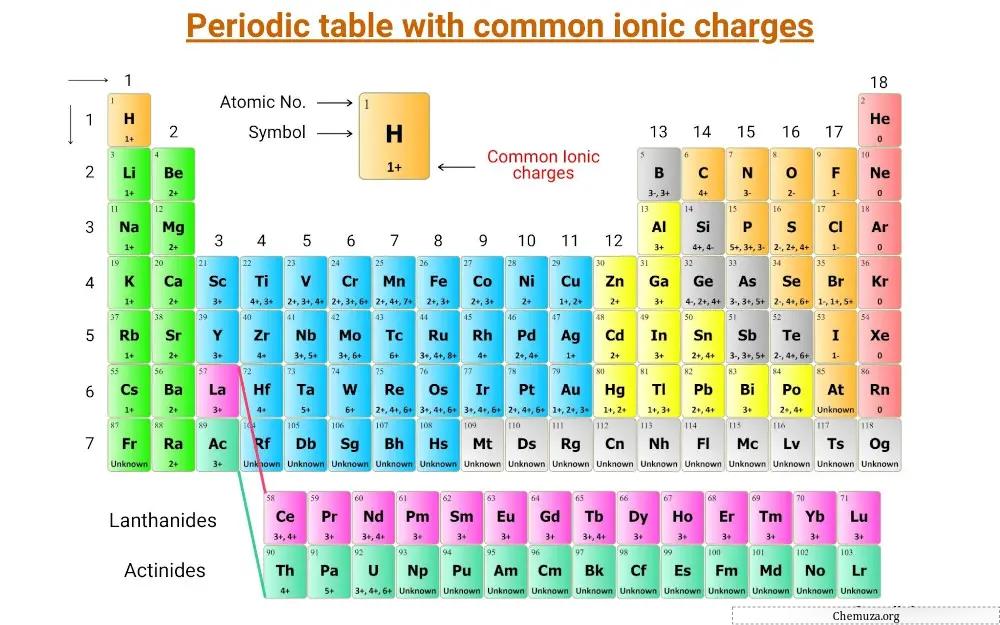
Dit is een periodiek systeem waarop de gemeenschappelijke ionische ladingen van de elementen worden vermeld.
Je kunt hier ook HD-afbeeldingen downloaden;
Lijst met elementen met hun gemeenschappelijke ionische ladingen
Gemeenschappelijke ionische ladingen van de elementen worden weergegeven in de onderstaande tabel.
| Atoomnummer van het element | Ion | Gemeenschappelijke kosten) |
| 1 | Waterstofion | 1+ |
| 2 | Helium- ion | 0 |
| 3 | Lithium- ion | 1+ |
| 4 | Beryllium- ion | 2+ |
| 5 | Boriumion | 3-, 3+ |
| 6 | Koolstof- ion | 4+ |
| 7 | Stikstof- ion | 3- |
| 8 | Zuurstof ion | 2- |
| 9 | Fluorion | 1- |
| tien | Neon- ion | 0 |
| 11 | Natriumion | 1+ |
| 12 | Magnesium- ion | 2+ |
| 13 | Aluminium ion | 3+ |
| 14 | silicium ion | 4+, 4- |
| 15 | Fosfor- ion | 5+, 3+, 3- |
| 16 | Zwavelionen | 2-, 2+, 4+, 6+ |
| 17 | Chloor- ion | 1- |
| 18 | Argon- ion | 0 |
| 19 | Kalium- ion | 1+ |
| 20 | Calciumion | 2+ |
| 21 | Scandium- ion | 3+ |
| 22 | Titanium- ion | 4+, 3+ |
| 23 | Vanadium- ion | 2+, 3+, 4+, 5+ |
| 24 | Chroomion | 2+, 3+,6+ |
| 25 | Mangaan- ion | 2+, 4+, 7+ |
| 26 | ijzer ion | 2+, 3+ |
| 27 | Kobaltion | 2+, 3+ |
| 28 | Nikkel- ion | 2+ |
| 29 | Koperion | 1+, 2+ |
| 30 | Zink- ion | 2+ |
| 31 | Gallium- ion | 3+ |
| 32 | Germanium- ion | 4-, 2+, 4+ |
| 33 | Arseen- ionen | 3-, 3+, 5+ |
| 34 | Selenium- ion | 2-, 4+, 6+ |
| 35 | Broom- ion | 1-, 1+, 5+ |
| 36 | krypton- ion | 0 |
| 37 | Rubidium- ion | 1+ |
| 38 | Strontium -ion | 2+ |
| 39 | Yttrium -ion | 3+ |
| 40 | Zirkonium -ion | 4+ |
| 41 | Niobium- ion | 3+, 5+ |
| 42 | Molybdeen- ion | 3+, 6+ |
| 43 | Technetium -ion | 6+ |
| 44 | Ruthenium -ion | 3+, 4+, 8+ |
| 45 | Rhodium- ion | 4+ |
| 46 | palladium- ion | 2+, 4+ |
| 47 | zilver ion | 1+ |
| 48 | Cadmium- ion | 2+ |
| 49 | Indium- ion | 3+ |
| 50 | Tin- ion | 2+, 4+ |
| 51 | Antimoon- ion | 3-, 3+, 5+ |
| 52 | Telluur- ion | 2-, 4+, 6+ |
| 53 | Jodium-ion | 1- |
| 54 | xenon- ion | 0 |
| 55 | Cesium- ion | 1+ |
| 56 | Barium- ion | 2+ |
| 57 | Lanthaan -ion | 3+ |
| 58 | Cerium- ion | 3+, 4+ |
| 59 | Praseodymium- ion | 3+ |
| 60 | Neodymium- ion | 3+, 4+ |
| 61 | Promethium- ion | 3+ |
| 62 | Samarium- ion | 3+ |
| 63 | Europium- ion | 3+ |
| 64 | Gadolinium- ion | 3+ |
| 65 | Terbium- ion | 3+, 4+ |
| 66 | Dysprosium- ion | 3+ |
| 67 | Holmium- ion | 3+ |
| 68 | Ion Erbium | 3+ |
| 69 | Thulium- ion | 3+ |
| 70 | Ytterbium- ion | 3+ |
| 71 | Lutetium -ion | 3+ |
| 72 | Hafnium- ion | 4+ |
| 73 | Tantaal- ion | 5+ |
| 74 | wolfraam ion | 6+ |
| 75 | Renium- ion | 2+, 4+, 6+, 7+ |
| 76 | Osmium- ion | 3+, 4+, 6+, 8+ |
| 77 | Iridium- ion | 3+, 4+, 6+ |
| 78 | Platina- ion | 2+, 4+, 6+ |
| 79 | Gouden ion | 1+, 2+, 3+ |
| 80 | Kwik- ion | 1+, 2+ |
| 81 | Thallium -ion | 1+, 3+ |
| 82 | Lood -ion | 2+, 4+ |
| 83 | Bismut- ion | 3+ |
| 84 | Polonium- ion | 2+, 4+ |
| 85 | Astatine -ion | Onbekend |
| 86 | Radon-ion | 0 |
| 87 | Francium- ion | Onbekend |
| 88 | Radium- ion | 2+ |
| 89 | Actinium- ion | 3+ |
| 90 | Thorium- ion | 4+ |
| 91 | Protactinium- ion | 5+ |
| 92 | Radon-ion | 3+, 4+, 6+ |
